Along with RT-PCR screening, some experts argue that CT scans can be utilized to diagnose COVID-19. Discover more here concerning what to do if an individual starts experiencing signs. For online updates on the most recent developments pertaining to the unique coronavirus as well as COVID-19, click on this link. Larger, independent tests of the examination in medical setups would certainly be needed prior to the examination could be extensively embraced by healthcare providers. " The sensitivity of the COVID-19 Ag Respi-Strip is too low to meet the demands of a desktop conveyor frontline standalone triage examination, and also this restriction needs to be clearly interacted," they write.
This is estimated by optimum probability evaluation under the presumption that the number of viral RNA copies in the reaction input adheres to a Poisson circulation which each particle has the exact same likelihood of being enhanced. INSIGHT stage 1 with a fluorescence readout achieves an estimated LoD-95 of 46.6 [95% confidence period, 37.8 to 56.8] copies per 20 μl of response (Fig. 5C). The high variety of effective amplifications at low copy viral input (50 copies per response in Fig. 5A as well as 100 copies per response in fig. S4) suggests that the percentage of the barcodes that may disrupt the NASBA reaction is very low. Independently, INSIGHT stage 1 with dipstick readout has an LoD-95 of 75.8 (95% CI, 24.9 to 234) duplicates per 20 μl of reaction, which is computed using reactions displayed in Fig. 4C, we determined that the NGS sequencing alone has an LoD-95 of 80.3 duplicates (95% CI, 37.7 to 197) per 20 μl of reaction.
The 2021 Minnesota State Secondary School All Hockey Hair Group Includes Lots Of Beautiful Circulation.
The Division of Wellness and also Social Care claimed the Innova lateral flow gadgets detect over 95% of people with high viral load, one of the most contagious situations who are most likely to spread the virus additionally. Until lately, the examinations were made use of for mass testing particularly setups, such as schools. Nonetheless, previously this month the government revealed that LFTs would certainly be provided for all grownups to take twice a week. A swab is taken from the rear of the nose or throat as well as offers results a little like a pregnancy test, with a red line appearing if coronavirus proteins are found and also a 2nd line that indicates that the examination is functioning. The researchers conclude that, in a healthcare facility or health care setting, people that examine favorable might be quarantined and also obtain instant care, while those that test negative would certainly require to await the results of a conventional laboratory test. Significantly, when the scientists checked examples containing other microorganisms, including those that cause pneumonia, the acute rhinitis, as well as flu, none examined favorable.
- An uniqueness of less than 100%, i.e. the presence of false positives, elevates a different trouble which we will not pursue.
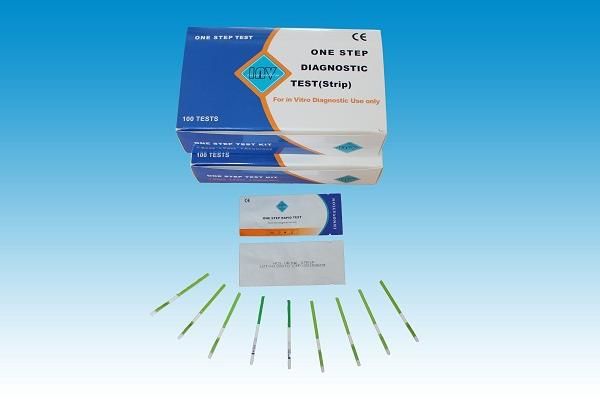
- These tests are extensively made use of in medical diagnostics for house screening, point of care testing, or lab usage.
- On July 31, the program awarded $248.7 million in contracts to 7 firms, whose modern technologies leveraged NGS, CRISPR, nucleic acid, as well as viral antigen platforms to recognize the infection.
- Somebody could increase- or perhaps triple-check their test results over a number of days.
- A person with COVID-19 may be transmittable 2 days prior to beginning to experience symptoms.
2 ELISA-based examinations, EDI IgM and also the Euroimmun IgG, were utilized for semiquantitative evaluation of IgM and also IgG degrees, respectively, of the examples evaluated with the LFAs (Fig. 2a). The EDI IgM ELISA had 35 false-negative results amongst the 40 convalescent plasma samples examined. Euroimmun IgG ELISA screening caused one false-negative result, which was IgG unfavorable by just about 3 of the LFAs evaluated. Both ELISAs produced one false-positive result in testing the prepandemic samples, as well as both of these were gotten for different examples.
Bu Today: As These Tests Multiply, How Will Our Lives Change? Will We Be Able (And Permitted) To Visit Restaurants?
The screening program has actually been created to lower the chain of transmission of the virus at the workplace. Under the programme, workplaces that remain open can develop targeted on-site screening, allowing employers to on a regular basis test staff members which, incorporated with social distancing as well as other preventative measures, must assist avoid break outs of the infection at the office.
Nevertheless, Mologic is likewise aiming to collaborate with US-based makers and also commercialization companions to take the tests via the FDA's Emergency situation Use Authorization procedure as well as range up production right here. The company expects that prototype assays will certainly be ready for recognition within weeks and launched with regulative authorizations, including CE noting and Globe Health and wellness Company Emergency Situation Use Listing, after 3 months. In the previous week a growing listing of diagnostic developers leveraging PCR technology has actually obtained Emergency Usage Permission from the United States Food and Drug Administration to identify the SARS-CoV-2, the coronavirus that triggers COVID-19. Keeping over the counter tests readily available at home can permit people to be prepared when it matters. People can feel more confident regarding going to events, seeing loved ones, or taking a trip when using this examination for COVID-19 testing. With the help of Randox Health, EasyJet is offering home tests for ₤ 72 (rather than ₤ 120).
E25Bio has actually sent for EUA a rapid antigen test to be offered by prescription and remains in the procedure of supplementing that entry with a submission for non-prescription use. Chouta claimed that the test meets the efficiency requirements for home testing set out in the FDA template.
Open Door To Bioworld Coronavirus Articles.
When incorporated with a lot more sensitive and specific tests, they can stop further episodes. LFTs have been used for mass testing for COVID-19 worldwide and enhance other public wellness measures for COVID-19.
Organizations across the country were invited to take up the Federal government's free work environment testing programme, making use of the quick lateral flow tests which can offer a result in thirty minutes. Any kind of examination you take only tells you if you have viral product in your nose at one point in time. We are fairly certain that really sensitive qPCR, like we do at BU, can capture individuals who are at the very beginning of the infectious stage. The Lucira test likewise amplifies viral RNA, however the test itself is less sensitive than laboratory-based qPCR, so it may not catch an infection before the moment when a person can spread out the virus. 2 months back, the Fda released an Emergency situation Use Authorization for the first quick COVID-19 diagnostic examination that can be used in your home.
You utilize an eye dropper to dispense six declines of chemical right into a tiny hole in the card; after that you put a swab after you've run it around in both nostrils. Rotate the swab counterclockwise, fold the card to bring the test strip in contact with the swab, which's it. Fifteen mins later, a positive outcome will certainly turn up as a faint pink line. After attempting all the tests, I am not preparing to invest in using them consistently.
However we would certainly be much better off refining call mapping," he told The Tab. The companies will certainly work in parallel to finish the examination prior to field testing. GE Health care Life Sciences will support Sona through their researches as they work to obtain their rapid-response Covid-19 lateral flow examination introduced right into markets as swiftly as feasible. Sona Nanotech Inc. and GE Healthcare Life Sciences will jointly complete examination advancement of the Sona Covid-19 Coronavirus fast response lateral flow test, as well as will utilize GE Medical care Life Sciences' Fast Circulation High Efficiency Membrane in manufacturing of the test. Some scientists arguethat if testing prevails sufficient and duplicated regularly, reduced accuracy is not a trouble. The disagreement is partially that repeat examinations will certainly catch some missed infections-- it's a volume video game. According to a version released just recently by the National Bureau of Economic Study, rapid tests might save tens of countless lives in the UNITED STATE as well as boost GDP even at reduced levels of accuracy and even where compliance with separating is reduced.
An even more extensive technique is the only way to restore some normalcy. One grievance is that the federal government hasn't made its proof base for the screening plans clear. Never one to undersell an initiative, Johnson's strategies appear to be asking more of the examination than it was developed for. Innova's Instructions for Use handout states the examination is designated for individuals that have signs as well as who are in the first 5 days of those signs.